All-in-One Platform
Everything you need, right when you need it. View Quotes, Invoices, Monitor Production, review quality and access documents, all from an intuitive dashboard.
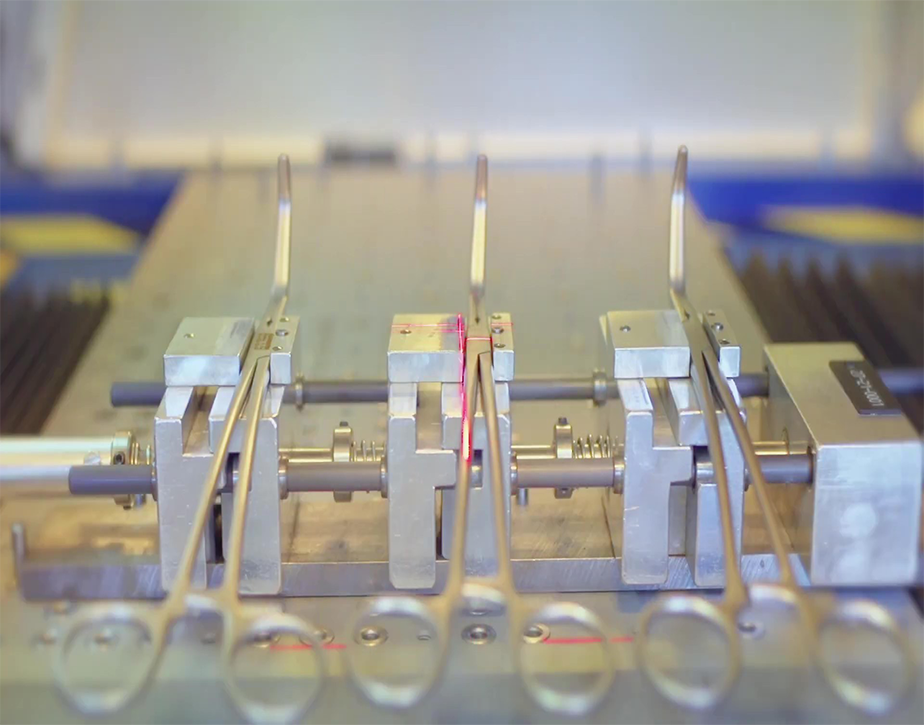
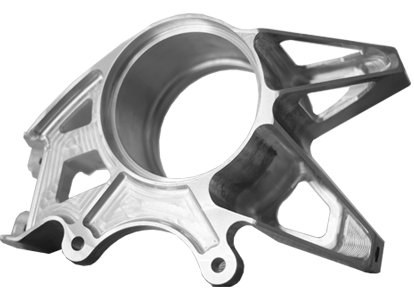
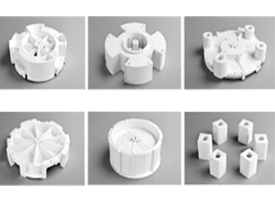
Production Updates
Detailed Inspection Reports & Photos
Centralized Access to Documents
Integrated Messaging